Research Group for Nano Structuring and Bio-Analytics (NASAN)
NASAN's main field of research is the development of methods for the quantitative analysis of biochemical and biological systems.
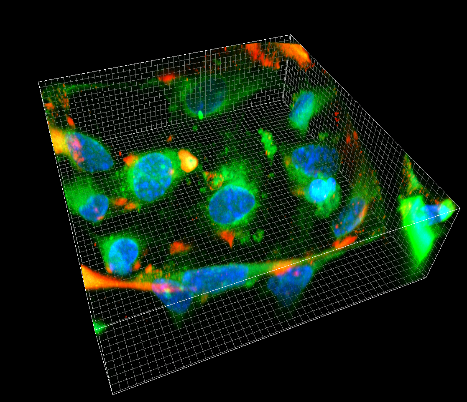
Our group has many years of experience in single-molecule fluorescence microscopy, atomic force microscopy and high-speed atomic force microscopy, quartz crystal microbalance and 2D/3D nanolithography. We have a cell culture laboratory (BSL 2) and a well-equipped standard chemical laboratory with facilities for protein analysis.
Method Development
NASAN focuses its expertise on single-molecule fluorescence microscopy, atomic force microscopy and high-speed atomic force microscopy, quartz crystal microbalance and 2D/3D nanolithography. We are interested in combining these nanotechnological methods to qualitatively and quantitatively study biological systems of varying degrees of complexity, from the dynamics of single proteins to the behavior of complex tissues.
3D nanolithography is the only tool that enables 3D structuring with structure sizes from micro to nanometers. We use our lithography systems for the modification of microfluidic channels, the structuring of 3D tissue scaffolds and for conductive structures. We also use maskless UV lithography for large-area 2D microprints.
Quantitative atomic force microscopy (AFM) and high-speed atomic force microscopy (HS-AFM) are used to investigate the topography, mechanical properties and molecular dynamics at the nanoscale. In particular, HS-AFM allows us to determine the structure and degree of oligomerization of proteins, the translation and conformational dynamics, as well as the binding kinetics that control the association/dissociation with binding partners. Quantitative AFM also allows us to characterize samples (from single proteins to cells and tissues) with respect to their quantitative nanomechanical and adhesion properties.
Multicolour 3D superresolution microscopy is a method to characterize biological processes in single cells nanoscopically. Using this technique and comparative 3D image analysis, it is possible to distinguish different states of a cell at the nanoscopic level, 3D single molecule tracking can quantify the dynamics of individual molecules within cells and quantitative analysis of the intensity of individual emitters can determine the abundance of certain proteins in cells.
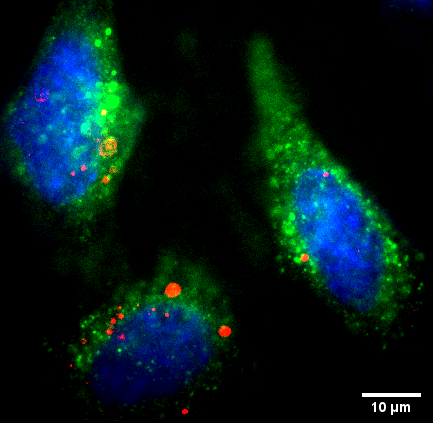
Quantification of Bio-nano-particles
We quantify different types of bio-nanoparticles (antibodies, high-density/low-density lipoprotein particles, extracellular vesicles, etc.) in terms of their interactions, their shape/geometry/oligomeric state, their lipid/protein composition as well as their cellular uptake and downstream effects.
We use quantitative atomic force microscopy to study the topography and mechanical properties of bio-nanoparticles (BNPs), use high-speed atomic force microscopy to directly visualize the molecular dynamics and underlying interactions, and quantify the abundance of fluorescently tagged proteins in BNPs. Multicolor 3D super-resolution microscopy in combination with quantitative 3D image analysis also allows us to image biological processes in individual cells nanoscopically and to compare different states of a cell at the nanoscopic level.
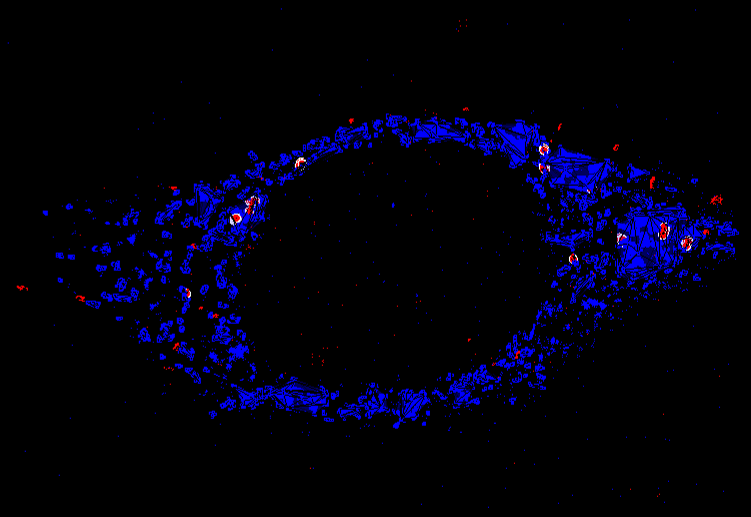
3D Tissue Scaffolds in Microfluidics
In NASAN, we develop microscopic 3D tissue scaffolds for 3D cell culture that fit into small microfluidic devices. In this way, we mimic complex systems such as the blood-brain barrier and use high-resolution fluorescence microscopy techniques to monitor transport processes between the different cell layers.
3D tissue scaffolds are printed using 3D multiphoton lithography and consist of functional polymers. This makes it possible to tune the mechanical properties, coat the structures with proteins and improve biocompatibility. For quantitative analysis, we combine microfluidics with multicolor, single-molecule sensitive fluorescence microscopy, which enables the visualization of molecular co-localization, 3D tracking of uptake events and quantification of molecular processes on the nanometer scale. Recently, we have developed a dual-channel microfluidic system that mimics the blood-brain barrier - a 3D scaffold used to study thrombus formation and for use as a 3D protein chip.

Protein Dynamics
We characterize the interplay of antibodies, complement proteins and Fc-receptors that trigger immunological effector functions.
We use different biophysical techniques to characterize the interaction of proteins involved in various immunological processes such as activation of the complement system and antibody-dependent cell-mediated cytotoxicity. Using high-speed atomic force microscopy, we can directly observe these processes on antigenic membranes with nanometer spatial and millisecond temporal resolution, while complementary single-molecule force spectroscopy, quartz crystal microbalance and surface plasmon resonance provide kinetic reaction rates, affinities, and interaction forces and energies.The combination of these methods allows us to characterize the respective molecular pathways in detail, create complex models of them and perform computer simulations to further deepen our understanding of the spatial and temporal relationships of proteins that trigger or inhibit a particular immune response. Our overarching goal is to use this knowledge to identify opportunities within the molecular signaling pathways where inhibitory factors or drugs can interfere with or enhance the actual process - and thus support the preclinical development of drugs to modulate the complement system and immune effector cells in the context of immunotherapies.

Contact
Research Group NASAN
Quick Links
I'll help you choose your study program.